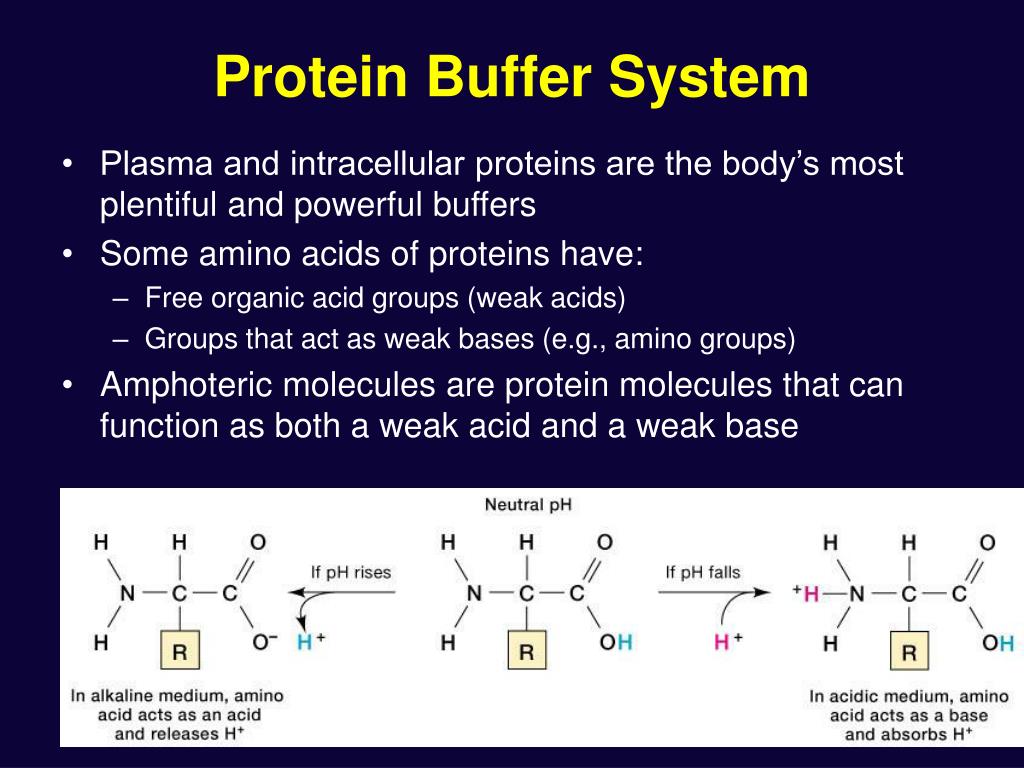
Buffer In Biology Example . identify the characteristics of acids. you should be aware that buffers play a critical role in almost all biochemical systems. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Identify the characteristics of bases. there are two classes of buffers which play crucial roles in the human body: Define buffers and discuss the role they play in human biology. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). (1) the bicarbonate buffer system and (2) the. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
from www.slideserve.com
you should be aware that buffers play a critical role in almost all biochemical systems. (1) the bicarbonate buffer system and (2) the. Define buffers and discuss the role they play in human biology. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. there are two classes of buffers which play crucial roles in the human body: for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). Identify the characteristics of bases. identify the characteristics of acids. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation
Buffer In Biology Example Define buffers and discuss the role they play in human biology. Identify the characteristics of bases. Define buffers and discuss the role they play in human biology. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). (1) the bicarbonate buffer system and (2) the. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. there are two classes of buffers which play crucial roles in the human body: the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. identify the characteristics of acids. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in.
From www.youtube.com
Introduction to buffers Water, acids, and bases Biology Khan Buffer In Biology Example the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Identify the characteristics of bases. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within. Buffer In Biology Example.
From www.hopaxfc.com
Biological Buffers Products Hopax Fine Chemicals Buffer In Biology Example for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Define buffers and discuss the role they play in. Buffer In Biology Example.
From users.highland.edu
Buffers Buffer In Biology Example Define buffers and discuss the role they play in human biology. you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. learn what biological buffers are, how they work and why they are important for. Buffer In Biology Example.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Buffer In Biology Example learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. there are two classes of buffers which play crucial roles in the human body: Identify the characteristics of bases. identify the. Buffer In Biology Example.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Buffer In Biology Example (1) the bicarbonate buffer system and (2) the. Define buffers and discuss the role they play in human biology. there are two classes of buffers which play crucial roles in the human body: you should be aware that buffers play a critical role in almost all biochemical systems. the buffer systems functioning in blood plasma include plasma. Buffer In Biology Example.
From www.youtube.com
Buffer action in the blood YouTube Buffer In Biology Example (1) the bicarbonate buffer system and (2) the. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. there are two classes of buffers which play crucial roles in the human body:. Buffer In Biology Example.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Buffer In Biology Example identify the characteristics of acids. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). there are two classes of. Buffer In Biology Example.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Buffer In Biology Example there are two classes of buffers which play crucial roles in the human body: Define buffers and discuss the role they play in human biology. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. Identify the characteristics of bases. (1) the bicarbonate buffer system and (2) the. you. Buffer In Biology Example.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Buffer In Biology Example (1) the bicarbonate buffer system and (2) the. Define buffers and discuss the role they play in human biology. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). Identify the characteristics of bases. the buffer systems functioning in. Buffer In Biology Example.
From www.researchgate.net
(PDF) Buffer Biology Buffer In Biology Example the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). there are two classes of buffers which play crucial roles in. Buffer In Biology Example.
From www.slideserve.com
PPT AcidBase Buffers PowerPoint Presentation ID496487 Buffer In Biology Example you should be aware that buffers play a critical role in almost all biochemical systems. there are two classes of buffers which play crucial roles in the human body: the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Define buffers and discuss the role they play in human biology.. Buffer In Biology Example.
From www.youtube.com
Acid, Base & Buffer in Biological systems YouTube Buffer In Biology Example identify the characteristics of acids. you should be aware that buffers play a critical role in almost all biochemical systems. learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. Identify the characteristics of bases. for example, a buffer can be composed of dissolved acetic acid (hc 2. Buffer In Biology Example.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists Buffer In Biology Example the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. for example, a buffer can be composed of dissolved acetic acid (hc 2 h 3 o 2, a weak acid) and sodium acetate (nac 2 h 3 o 2). (1) the bicarbonate buffer system and (2) the. there are two. Buffer In Biology Example.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer In Biology Example Define buffers and discuss the role they play in human biology. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. there are two classes of buffers which play crucial roles in the human body: (1) the bicarbonate buffer system and (2) the. for example, a buffer can be composed. Buffer In Biology Example.
From www.expii.com
Buffers — Definition & Overview Expii Buffer In Biology Example identify the characteristics of acids. (1) the bicarbonate buffer system and (2) the. Define buffers and discuss the role they play in human biology. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. you should be aware that buffers play a critical role in almost all biochemical systems. Web. Buffer In Biology Example.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers Buffer In Biology Example there are two classes of buffers which play crucial roles in the human body: the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Define buffers and discuss the role they play in human biology. Identify the characteristics of bases. the buffer systems functioning in blood plasma. Buffer In Biology Example.
From www.slideshare.net
Biology Keynote Buffer In Biology Example learn what biological buffers are, how they work and why they are important for maintaining optimal ph in. Define buffers and discuss the role they play in human biology. there are two classes of buffers which play crucial roles in the human body: the purpose of a buffer in a biological system is to maintain intracellular and. Buffer In Biology Example.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer In Biology Example (1) the bicarbonate buffer system and (2) the. identify the characteristics of acids. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. you should be aware that buffers play a critical role in almost all biochemical systems. for example, a buffer can be composed of. Buffer In Biology Example.